Final Mini Quiz guys :P Good Luck! Until we meet again….
Part A. MULTIPLE CHOICE QUESTIONS:
- The initial molecule formed by deamination of alanine enters the TCAcycle as
- Fumarate
- Oxaloacetate
- Glutamate
- Acetyl CoA
- Malate
- Succinate
- Which of the following is not a characteristic of carbohydrates in cells?
- Act as binding sites for proteins
- Organic catalysts
- Major structural components of plant tissues
- Energy stores for plants and animals
- Play a role in cell to cell recognition
- Which of the following describe(s) the peptide bond?
- Substituted amide linkage
- Formed in a condensation reaction
- Covalent bond between amino acids
- Soluble in water
Part B. STRUCTURED QUESTIONS:
- How would a pyruvate kinase deficiency affect haemoglobins affinity for oxygen?
- What structural aspect of TPP allows it to act as an electron sink?
- Why is the phosphoryl transfer from 1,3-bisphosphoglycerate to ADP in glycolsis called substrate level phosphorylation?
- Are enzymes always proteins, consisting of only amino acids? Why/Why not?
Posted in Science and Biochemistry
The Biochemistry and Molecular Biology of Programmed Cell Death
The Biochemistry and Molecular Biology of Programmed Cell Death.
Apotosis or programmed cell death can be described as “natural cell death”. Apotosis plays an important part in pathological and physiological processes. Programmed cell death occurs in two distinct phases. In the first phase, there is condensation in both the nucleaus and cytoplasm of the cell. This eventually results in the formation of small membranous fragments which contains cell organelles that are intact structurally. In the second phase however, the membranous fragments which were earlier in the first phase are phagocytised by their neighbouring cells. The question that remains is, how really this process occurs at the biological level.
Although it was difficult to obtain a clear indication as to what really happens at the molecular process of Apotosis, in 1980 a paper by Wyllie revealed that glucocorticosis induces rapid DNA fragmentation in the thymocytes of rats in vitro upon cell death. The link between DNA fragmentation and cell death is quite interesting. According to Wyllie, at the molecular level, DNA is fragmented at specific patterns, which results in DNA fragments which are the same length of the DNA surrounding the histones octamer in a nucleosome. This fact suggests that chromosome cleavage is taking place at the molecular level of Apotosis. DNA fragmentation is directly linked to cell death.
In Apotosis, there are also various intercellular signals which hints that Apotosis is taking place. One of the changes that occur at the intercellular level is the notable increase of calcium concentrations within the cell. This is important since the inhibition of calcium increase prevents stimulus induced cell death. Further, although it is still unclear as to what really is the role of calcium in Apotosis or programmed cell death, it is suggested that the increase of cellular calcium concentrations activates the fragmentation of DNA.
Protein Kinase A and Protien Kinase C are also important indicators that programmed cell death is taking place within the cell. Protein Kinase C promotes the negative regulation of cell death while protein Kinase A Increases cAMP concentrations with in the cell.
Another important intracellular indicators of Apotosis is the synthesis of RNA and proteins. This is of a great significance since programmed cell death is active and energy consuming. As a result, the synthesis of proteins are required for the processes that lead up to cell death. Interestingly however, although the synthesis of proteins and RNA are needed for Apotosis to occur, studies has also proven that inhibitors of protein/RNA synthesis does not only prevent programed cell death which is mediated by other signals but that it also induces programmed cell death themselves. This finding suggests that there may be various mechanisms which promotes programmed cell death and the preparation stages of Apotosis varies differently from cell to cell.
Altogether, Apotosis is an important process since it has the potential role of preventing or even curing many diseases. If programmed cell death is controlled, it can play an important role in the treatment of diseases which are caused by uncontrolled cell growth. For instance, in inflammatory reactions, there I s a large movement of neutrophils into the site where inflammation is taking place. In order to stop inflammation, neutrophils is removed from inflammatory sites via programmed cell death and identification by macrophages. Since the removal of these cells needs to be done without the releasing of the granular contents of the neutrophils and the activation of programmed cell death in neutrophils can prevent the injury of tissues which are/ can be inflamed.
Although many studies have been done in order to gain a clear understanding of Apotosis at the molecular level, it is still quite difficult to fully understand this marvellous process. As a result, there is still a great need to further research the in-depth process of Apotosis or programmed cell death if we really seek to gain a comprehensive understanding of this process.
References:
Robert A.Schwartzman, John A. Cilowski, “The Biochemistry and Molecular Biology of Programmed Cell Death”, Volume 12, Number 2 Pages 133-154
Posted in Uncategorized
Youtube Video Review 1
The controversy surrounding Black Holes and Wormholes – A review of SafeFriction’s video on “Black Holes and Wormholes”
Wormholes and black holes are one of the most controversial topics of science and have been so for the past few decades since they were discovered. This concept was first constructed by Einstein who with his partner Rosen, formulated the Einstein Rosen bridge through relativity laws, also known as the wormhole. A documentary released by SafeFriction on December 11th 2013, explored these two main concepts and opened up man intriguing ideas surrounding these phenomena, highlighting them through introductory graphics which made the viewer want to continue watching and captured their attention. Through visual stimuli the documentary took a ‘journey through the universe’ almost as though one was searching for the phenomena themselves.
Image credit: SafeFriction- https://www.youtube.com/watch?v=P1OBL5J_n98
Clifford Johnson of the University of Southern California made an analogy of wormholes to subway systems, a feature many people could relate to. Imagining this phenomenon as a pathway through which one can travel from one point to another essentially is the basis for the Einstein Rosen Bridge. However, according to Mark Morris and Gregory Benford, his laws did not originally allow for time travel or travel at all. This was surprising as most of us believe that this is the concept behind wormholes in the first place. However, Einstein’s theory suggested that wormholes are unstable and unable to maintain their pathway for more than a mere short time period. If something were to entre a wormhole, it would be crushed as both ends pulled apart and the wormhole separated at the centre of the pathway.
Michio Kaku after years of research suggested that wormholes could be made stable through use of antimatter this would hold it open for enough time that something may be able to pass through it. However the documentary opened up a frightening concept; one may know where a wormhole begins but not where it exits. That is, we may enter a wormhole and expect to come out at another point in space but may actually come out in the core of a star or worse. Another broad concept of wormholes is that of traveling to the future. Benford and Johnson stated that Einstein also made this idea achievable. Through experiments, clocks that moved on earth move at a slower time than clocks that were in space due to the lack of gravity. This suggests that moving through space one would be moving at a faster time than one moving on earth and therefore due to time differences it becomes possible to use a wormhole to enter a different time period, whether past or future.
Image credit: SafeFriction- https://www.youtube.com/watch?v=P1OBL5J_n98
However this as suggested by Johnson and Kaku can result in a paradox known as the “Grandfather Paradox” where a person could go back into the past and kill their grandfather thus ending their generational line. It may even be possible to meet a past relative through time travel. As absurd as these theories are, these scientists still consider them as theories until proven otherwise.
Image credit: SafeFriction- https://www.youtube.com/watch?v=P1OBL5J_n98
But what about black holes? They are areas of strong gravitational pull which can consume even light. Any object that gets too near to a black hole would be sucked in. Morris compared this phenomena to a fish swimming near a waterfall or an inverse fountain. As the fish nears the edge of the waterfall it becomes harder to swim away. If that fish enters the centre of the waterfall, it is past the point of return and therefore cannot leave. And as we stated earlier there is the plausibility of a white hole, possible due to Einstein’s theories of relativity. It is the reverse of a black hole, similar to a fountain where objects come out of them. Quasars were once thought to be white holes due to their great energies and ability to ‘throw out’ objects. However this was disproven as quasars are formed due to black holes. Ironically white holes are yet to be discovered and are a point of singularity where matter is ejected. Morris states that it is believed that the universe may have been formed by this phenomenon, essentially the Big Bang Theory. Benford raised a good point though that persons once believed black holes to be fictional but these were disproven, so who knows what may occur with white holes as well.
Image credit: SafeFriction- https://www.youtube.com/watch?v=P1OBL5J_n98
Surprisingly, the documentary highlighted that there are in fact two types of black holes: Stellar mass black holes and supermassive black holes. The latter is millions to billions the size of our Sun. It is stated that the Milky Way has one. However, all are considered nightmares. Kaku states, “They’re like check in hotels. Something checks in and nothing checks out”. It is said that ‘spaghettification’ occurs, that is, tidal forces stretch the object so much that everything is eventually destroyed, down to our cells. Sometimes black holes may pair up. Each becomes trapped by each other’s gravitational fields, eventually colliding. This creates a gravitational wave outwards carrying energy with it. Morris compares this to ripples formed from throwing stones in a pond, the ripples radiate to far ends of the pond.
Image credit: SafeFriction- https://www.youtube.com/watch?v=P1OBL5J_n98
In January 2007, US satellites observed a three way collision between quasars powered by supermassive black holes. Benford states that one of these could be ejected out becoming a rogue black hole while the other two collide. Kaku states that the deepest question concerning scientists and even us, is what lies on the other side of a black hole. According to Einstein, nothing can ever leave a black hole. However, a small black hole may allow for the phenomena to occur through quantum theory as they radiate energy as suggested by Stephen hawking. The documentary raises an interesting piece of information. Scientists are attempting to form mini black holes right here on earth.
Image credit: SafeFriction- https://www.youtube.com/watch?v=P1OBL5J_n98
In Switzerland we know that the Hadron collider particle accelerator was produced by scientists. It can combine a lot of energy together, so much that microscopic black holes could be formed in the collisons and thus be studied to determine how they work.
Image credit: SafeFriction- https://www.youtube.com/watch?v=P1OBL5J_n98
There is a fear that these would be able to escape and consume the earth but this fear was dismissed by Kaku and Timothy Hallman, as their microscopic size would not allow this to occur. The documentary ends by stating that physicists are still monitoring all of these phenomena, pushing the boundaries of Einstein’s laws. As “tickets to oblivion” and “unicorns of astronomy” these phenomena still continue to be pursued by scientists and hopefully in the coming decades our questions will be answered, but until then this documentary served to answer and relieve man of our questions, concepts and even worries while demonstrating a fantastic visual stimuli for all viewers. A recommended watch for all.
Image credit: SafeFriction- https://www.youtube.com/watch?v=P1OBL5J_n98
Posted in Uncategorized
Published Paper Review
The Biochemistry Behind Auxins As Plant Growth Hormones
Most of us have heard of the term ‘auxins’ at least once or twice in our lives, whether it was in the early years of high school or college. The term ‘auxins’ is always associated with plant growth and elongation, mainly because that is its purpose. Auxins are enzymes which inhibit the elongation of plant shoot tips so that growth may be facilitated. But just what is the biochemistry behind this action?
Indoleacetic acid which is the main auxin present in today’s plants has been found to be similar in structure to other substances responsible for plant growth. In fact, cabbage leaf growth was found to be solely controlled by indoleacetic acid. This information based on studies conducted and compiled by James Bonner was discovered through extraction of the pure auxin from plants in order to examine its activity. From examination of auxins of the oat coleoptile, they were found to mainly or almost completely consist of indoleacetic acid. This suggests so far that the biochemistry behind plant growth may be dependent on this unique substance
But what exactly is indoleacetic acid? Formally called indole-3-acetic acid, it is a plant hormone that key in shoot and root growth in plants. As to how it moves through developing plants to their tips, this accomplished through importers such as (AUX 1) and efflux pumps. These simply act as pathways through which the auxins can pass in order to accumulate at the shoot or root tip when required. Indoleacetic acid has also been observed to be active when a plant is damaged, suggesting that it may be present at sites of plant wounds.
As a hormone, indoleacetic acid was found to be easily degraded by acids, specifically HCl and oxidizing agents. According to Bonner, the presence of oxygen had a reactive action on the auxin. As expected the enzyme indoleactic acid oxidase also easily destroys this auxin.
Based on Bonner’s findings, the indoleacetic acid is initially formed by tryptophan, where a substance known as an indole acetaldehyde forms and acts as an intermediate in the overall reaction. This acetaldehyde is oxidized into an acid. Enzymes further react with this aldehyde converting it to indole acetic acid. This serves as the auxin we know as indoleacetic acid. This acid has a six ringed structure which has multiple stereoisomers, 2,4-di-substituted indoleacetic acid being an inactive isomer.
Their aromatic ring structure allows them to undergo substitution reactions where groups such as methyl to substitute H ions on its structure on either carbons 2 or 4. Depending on where each substitution occurs, and whether a substrate molecule is the ‘substitute’, the auxin’s activity may increase or decrease. According to an extract by James Bonner, this discovery of the hormone’s ability to undergo substrate binding and activity similar to enzymes opened the study of auxins and other plant growth hormones to a lot more interest factors such as the possibility of active binding sites on their structures. Interestingly after investigating further, auxins were found to undergo inhibition similarly to the way in which enzymes do. Reactions between indoleacetic acid and inhibitor and indoleacetic acid on the rate of plant growth were carried out and graphs constructed similar to the Lineweaver Burk plots of enzymatic activity but with various results and x-axis, y-axis values.
Although experiments are still to be carried out in order to discover more about auxin activity, it can be considered a very diverse substance. Not only a plant growth hormone, but an active stereoisomer with biochemistry surprisingly similar to that of enzymes, the study of the biochemical background of auxins has opened up a unique history of the hormone, paving the way for the possible discovery of new underlying plant reactions or even of further plant growth hormones unknown to us.
References:
James Bonner, Robert S. Bandurski, ‘Studies of the Physiology, Pharmacology and Biochemistry of the Auxins, Pages 59-70, Website http://authors.library.caltech.edu/1646/1/BONarpp52.pdf
Reeta Prusty et al, January 29th 2004, “The plant hormone indoleacetic acid induces invasive growth in Saccharomyces cerevisiae”, Proceedings of the National Science Academy of The United States of America, Volume 101, Number 12, Pages 4153-4157, Website http://www.pnas.org/content/101/12/4153.long
Posted in Uncategorized
Youtube Video Review:- Multiple Sclerosis: How can we stop disability progression? The story of restoring myelin
Multiple Sclerosis: How can we stop disability progression? The story of restoring myelin…
Video By: Susan van Rensburg
Channel Youtube: Brain Biochem
In Multiple Sclerosis, myelin in nerve cells of the brain and spinal cord are damaged. This causes a lack of communication between certain body parts which result in mental, physical and psychiatric problems. This is a disability. The test for the amount of disability uses a scale (EDSS) which shows the amount of disability over time. 0.0 on the scale means that there is no disability whereas 10.00 mean death. (Readings of 0-3 indicates a benign case.)
There is a myth that states that disability progression is inevitable. However, in this video, Dr. Rensburg aims to investigate if it can be reversed. In some studies carried out, it was found that some persons show progress whilst others don’t. Why? Myelin is the fatty covering of nerve axons. In Multiple Sclerosis however, the myelin is damaged. Thus, Multiple Sclerosis is based on the source that manufactures myelin, oligodentrocytes. These are round cells with big nuclei and numerous dendrites. According to Dr. George Bartzokis, the brain ‘intranet’ depends on synchrony and speed provided by myelin. Thus, the thicker the myelin, the more effective it is. As a result, the brain is able to function better. Myelin increases processing ability of the brain. It also expands bandwidth, that is, the number of action potentials transmitted per unit time due to connectivity. Myelin is indispensible for developing higher cognitive functions which are dependent on later myelinating oligodendrocytes. Thus the oligodendrocytes are able to keep making myelin according to need.
At optimal conditions for myelination, oligodendrites produce microglya. (The immune cells in the brain are at rest.) If the oligodendrocytes don’t receive proper nutrients or is exposed to unfavorable conditions such as smoke causes the oligodendrocyte to shrivel and eventually die. The microglya approach and investigate the conditions of the oligodendrocytes. The myelin as a result becomes dysfunctional as the axons are exposed. As a result, the oligodendrocyte precursor cells wake up. The microglya remove and digest dead oligodendrocytes. They clean up the axon by removing dysfunctional myelin myelin and they donate iron to oligodendrocyte precursor cells. This allows them to mature into new oligodendrocytes. This results in clinical restoration of the myelin on the axion which leads to restoration of the functions; this is how paralysed persons are able to walk again. For oligodendrocytes to work properly there must be optimum blood flow to bring nutrients to it. Microglya removes debris thus causing inflammation to peripheral immune cells. B and T cells are then called to assist. (cortizons are needed to control inflammation, if it is not treated, axons become damaged.)
To conclude, it was found that smoking, stress, bad diet, vitamin B 12 deficiency, iron deficiency and vitamin D deficiency cause an increase in EDSS as opposed to persons who did not experience these risk factors. Thus, disability progression can be stopped if the risk factors mentioned above are controlled. Thus myelin can be restored. Quite an interesting video and I highly recommend that you view it.
video used:
Posted in Uncategorized
Nucleotides and nucleic acids
NUCLEOTIDES AND NUCLEIC ACIDS:
Nucleotides are made up of a base, sugar and phosphate.
Figure 1 represents a nucleotide.

Nucleic acids are composed of carbon, hydrogen, oxygen, nitrogen and phosphorous but it is does not contain sulphur.
Figure 2 represents the structures of deoxyribose and ribose respectively.

Pyrimidines
Important pyrimidines include uracil, thymine and cytosine. Thymine is found in DNA, uracil is found in RNA and cytosine can be found in both DNA and RNA. Uracil and thymine are structurally similar compounds the chemical composition difference is responsible for uracil replacing thymine in RNA.
Purines
Important purines include adenine and guanine. Both of these compounds are found both in DNA and RNA and are referred to as the principal purines.
Figure 3 above represents the synthesis of a nucleotide.

DNA replication:
In DNA replication two DNA polymerases collaborate to copy the leading strand template DNA and the lagging strand template DNA. The DNA polymerase temporarily releases the lagging strand template DNA. As the DNA helicase continues to unwind the parental DNA the primase becomes activated and synthesizes a short RNA primer on the growing lagging strand. The DNA polymerase binds to the DNA again and becomes locked in by the clamp. The polymerase uses the RNA primer to begin making a copy of the short strand lagging DNA. The polymerase stalls when it reaches the RNA primer of the preceding okazi fragment and the entire cycle repeats.
Figure 4 illustrates the process of DNA replication.

AMP (adenosine mono phosphate) is used as a monomer in DNA and RNA. ADP ( adenosine di phosphate) is a very important organic compound and it is used for metabolism and also necessary for the flow of energy in living organisms. ATP ( adenosine triphosphate) it is referred to as the energy currency molecule and it is used to transport chemical energy within cells for metabolism.
Figure 6 shows AMP, ADP & ATP.
ATP is a very important molecule hence it will be discussed in detail. ATP is molecule that is present in the cells and it allows for quick and efficient access to energy whenever it is required by organelles in the cell. It is a type of chemical energy which is released when the chemical bonds are broken. When the bond is broken between two phosphate groups chemical energy is released. One of those phosphates is donated to the process that requires the energy. The process can now do the task that was required of it. After that has taken place two phosphate groups are remaining so the compound is no longer ATP it becomes ADP. If this molecule is to be used again it needs to be recharged. This can be recharged by the mitochondria, the ADP molecule goes to the mitochondria and it recharges the ADP by adding a phosphate molecule to it and it now becomes ATP again.
Nucleic Acids
It is essential acids that are present in the human body without it humans would not be able to survive. Nucleic acids are made up of nucleotides, when there is a sufficient amount of nucleotides present then a nucleic acid will be formed. The most common nucleic acid is DNA(deoxyribonucleic acid). DNA is made up of two strands of nucleotide. Nucleotide three main ingredients are nitrogenous base, sugar and phosphate.
Firstly nucleic acids are used to make protein this is the job of the DNA and RNA, secondly the make up the genes in the human body these genes are passed on.
Figure 7 represents the structures of DNA and RNA.
There are three major forms of DNA they are all double stranded and interact via complementary base pairing. These are termed as A- form-this is favoured by RNA, B- form-standard DNA double helix under physiological conditions and Z- from DNA-this is usually a laboratory anomaly.
Figure 8 represents A form, B form and Z form DNA.
DNA vs RNA
DNA is very important it codes for your traits. RNA carries the genetic message out to the cells so they could start producing proteins. Both DNA and RNA are nucleic acids. DNA has a double helix shape, DNA has bases which code for the traits these are as follows adenine, guanine, thymine and cytosine. Adenine binds to thymine and guanine binds to cytosine this is referred to as complementary base pairing. DNA is found in the nucleus. RNA also has four bases, it has all the same bases as DNA except for thymine is replaced with the base uracil. RNA starts in the nucleus but it travels out of the nucleus, there are three types of RNA firstly there is messenger RNA they carry the message to the cells. Secondly there is transfer RNA its job is to transfer the message and the last type of RNA is ribosomal RNA this is involved in the protein making.
References
http://www.youtube.com/results?search_query=nuceic+acids
http://www.youtube.com/watch?v=bbtqF9q_pFw
http://library.med.utah.edu/NetBiochem/pupyr/pp.htm
Posted in Uncategorized
What is TCA?
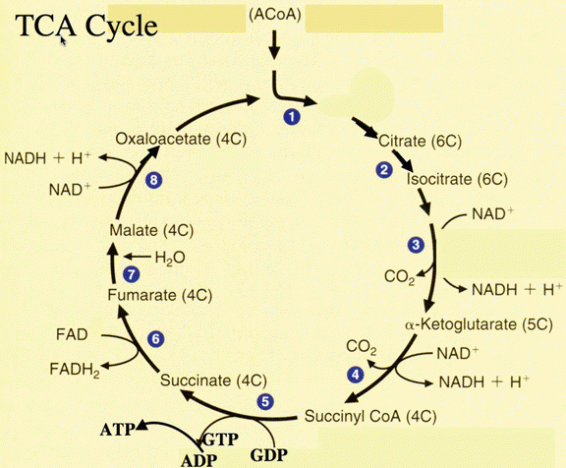
TCA:
The citric acid cycle, more commonly known as the Krebs cycle is an eight step process involving the oxidation of acetyl coenzyme a to carbon dioxide. The process is deemed a ‘cycle’ as oxaloacetate which is used to initiate the cycle, is regenerated once more in the final step of the reaction thus restarting the entire process again. The cycle is denoted below:
Image credit: http://www.unm.edu/~lkravitz/Exercise%20Phys/tcacycle.html
So where does it TCA occur?
The citric acid cycle occurs in the mitochondria. This is because mitochondria contain all of the enzymes necessary for the citric acid cycle to occur; that is both within the cycle and also for synthesis of ATP through oxidative phosphorylation which is observed in glycolysis.
Image credit: http://www.pixton.com/uk/comic/jfth65jv
This cycle involves a series of hydration, dehydration, rehydration, hydrogenation, dehydrogenation and phosphorylation reactions. Initially acetyl coenzyme a donates its acetyl group (CH3CO) to oxaloacetate catalysed by citrate synthase in order to form citrate in a condensation reaction.
CH3COSCoA + OCOO–CCH2COO– →COCH2COO–COHCOO–CH2COO–
Oxaloacetate is a four carbon compound while acetyl CoA is a two carbon compound. Therefore it is expected that citrate will be a six carbon compound. This reaction is known as a Claisen condensation reaction.
Claisen condensation reaction:
During this reaction, a carbanion is stabilised by the carbonyl of an adjacent ester, generally a thioester. A thioester is a product of esterification with the addition of a sulphur atom on one adjacent carbon atom thus the final molecule has a functional group C-S-CO-C
In TCA, this Claisen condensation reaction occurs as the thioester acetyl coenzyme a is reacting with oxaloacetate which is a ketone and acts as the carbanion in the reaction.
In step 2 of TCA, the citrate formed is reversibly converted to isocitrate catalysed by the enzyme aconitate hydrase in a dehydration/rehydration reaction. In this step water is initially lost in order to convert citrate to cis-aconitate, an intermediary tricarboxylic acid. Water is then reversibly re-added to the cis-aconitate in order to convert it to the isocitrate.
Steps 3 and 4 involve oxidative decarboxylation where oxidation of citrate to alpha-ketoglutarate catalysed by the enzyme isocitrate dehydrogenase occurs. The alpha-ketoglutarate is then converted to a thioester, succinyl coenzyme a and carbon dioxide by a dehydrogenase complex. In this reaction NAD+ is directly used in the cycle, and acts as the electron acceptor.
As succinyl coenzyme a is a thioester, like acetyl conenzyme a, it can also undergo a Claisen condensation reaction. However this DOES NOT occur. Instead succinyl CoA undergoes a substrate level phosphorylation with ATP as a reaction molecule. The ATP here serves to conserve the energy of the succinyl CoA thioester bond.
How is the energy of a thioester (like succinyl coenzyme A) conserved by ATP?
After phosphorylation occurs, the phosphoryl group formed after the enzyme catalysed reaction binds with ADP to form ATP. Since this ATP forms as a result of oxidative decarboxylation of the alpha-ketoglutarate, it is classified as a substrate level phosphorylation which refers to formation of energy only through transfer of a phosphate group.
Image credit: http://weheartit.com/entry/107067898
Steps 6-8 involve subsequent dehydrogenation and hydration reactions. Initially the succinate is oxidised to fumarate by succinate dehydrogenase. In this reaction hydrogen H2 is removed from succinate.
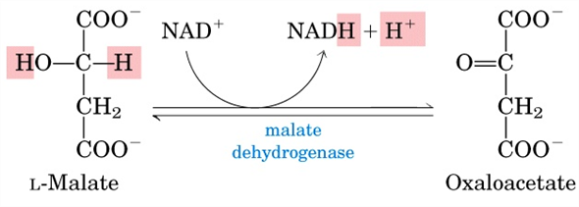
Step 7 involves hydration of the fumarate to malate. This is a reversible reaction and addition of an OH– and H+ ion to the trans bond of the molecule occurs in order to form malate.
The final step is oxidation of the malate to oxaloacetate by L-malate dehydrogenase. NADH is also formed in this reaction as H2 is removed and adds to NAD to form NADH and an extra H+ ion.
Image credit: Lehninger Principles of Biochemistry 5th Edition Page 647
So we know that these reactions occur in the mitochondria of a cell due to the presence of all the required enzymes, however what happens to substances like NADH and FADH2 that forms as byproducts of these reactions?
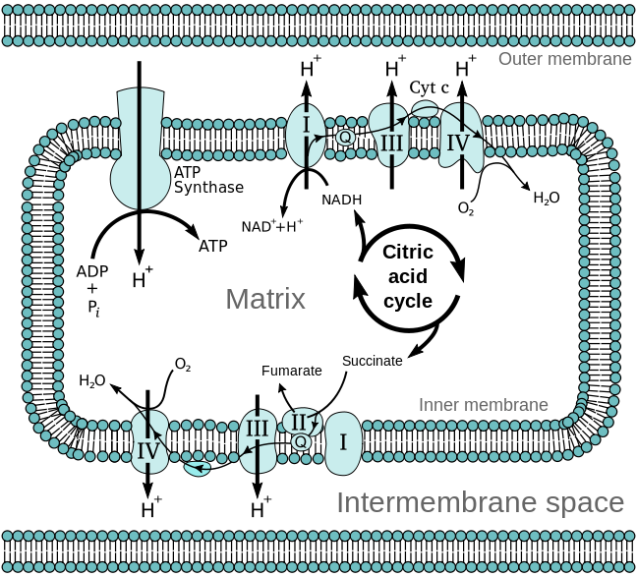
This is where the electron transport chain or ETC comes in.
Image credit: http://chemwiki.ucdavis.edu/Biological_Chemistry/Metabolism/Electron_Transport_Chain
These chains of reactions oxidise these byproducts to form ATP. This occurs through the transfer of electrons through NADH and FADH2 along with electron carriers along the chain. As electrons pass along the chain, hydrogen ions are released and since this process occurs within the mitochondria, they make their way into the intermembranal spaces within the mitochondria. As more hydrogen ions accumulate in the intermembranal space, the ratio of H2 ions in the matrix of the mitochondria to that in the intermembranal space becomes one-sided. This imbalance causes the enzyme ATP synthase to be activated and begin an ADP phosphorylation reaction thus forming ATP.
These reactions including TCA give a better indication of why the mitochondria are considered powerhouses of the cell as ATP is generally the main products of many of the reactions occurring within them.
Posted in Uncategorized
LIPIDS pt 1-The big fat truth!
What’s the deal with fat though? It’s a pain to lose and a joy to gain. Lipids are fatty acids that are a source of energy as it functions as an energy reserve where excess energy from carbohydrates and proteins are stored as triacylglycerols/triglycerides in the adipose tissue. Adipose tissue is stored under the skin and accounts for the gaining of weight. I wonder if you knew that before reading this blog…hmmm, I didn’t until I started this blog. That’s the thing about having to do this blog, you end up learning additional useful facts. Sometimes they are mentioned in the lectures and sometimes one has to take the initiative to do the additional work.

As I was saying, lipids function as an insulator for the body as the fat stores build up and lipids also function as a mechanical insulator as it protects vital organs in the body. These stores however can be tapped into when the body experiences an energy deficit. When this occurs the body begins to draw fat from the adipose tissue to suffice itself resulting in a reduction in adipose tissue. This is basis of exercise whereby the body requires large amounts of energy and uses the energy stored as fat under the skin. Thus, weight loss occurs. Cool huh? Yess!!!!
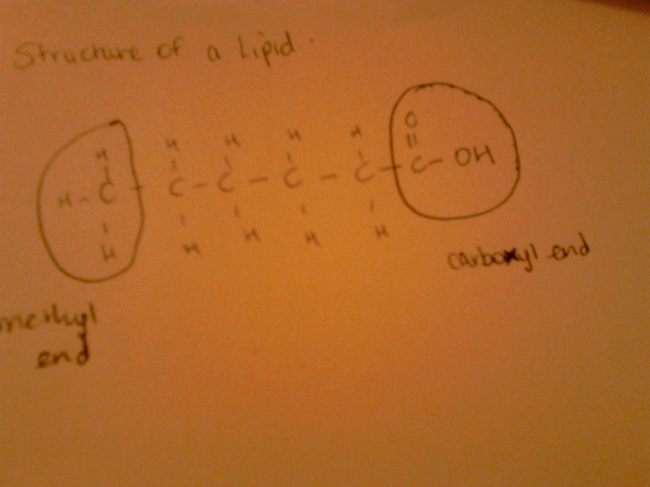
Lipids are used in the formation of cell membrane; it is involved in steroid hormones as well as it supplies essential fatty acids to the body. Essential Fatty Acids are required by the body through diet as the body is unable to manufacture carbon-carbon double bonds before the carbon 9th atom form the methyl group. This brings me to explaining the structure of a lipid molecule; take a look at the images below! Lipids can be in the form of fats or oils. These fats for example, margarine, are solids at room temperature due to their singly bonded carbon atoms throughout the molecule. Oils for example from plants are liquid at room temperature due to the carbon-carbon double bonds (C=C). Don’t be alarmed by the chemistry here, it’s simple. Looking at the lecture that our lecturer posted online, it was a piece of cake to grasp the concepts.
One thing in this section of the course that really stood out to me was the unsaturated molecule oleic acid and its functions. It really blew my mind. Here’s what it does! Oleic functions as an alert to other organisms when an insect dies. It signals members of the same species to move the body of the decaying corpse. It also signals the other insects to stay away from the body as it may have diseases.
An important part of lipids is delta designation which I was able to grasp easily after it was explained quite precisely in the lecture. Basically, you count the number of C atoms excluding the C atom from the carboxyl functional group of course. This is then flowed by counting the number of double bonds in the molecule excluding the double bond from the carboxyl group. This is the ratio. Now, to determine delta designation, we simply state the C atom holding the double bond from the carboxyl end.
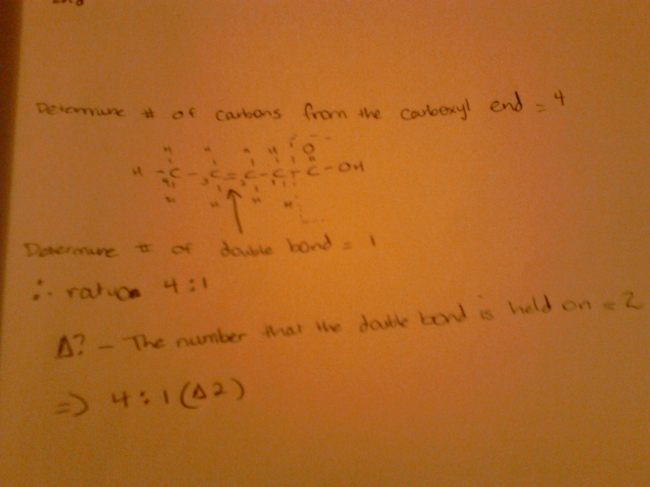
In fatty acids, as the chain length increase, the melting point increases as more energy is required to break all the bonds in the molecule. Solubility however, decreases as the length increase as the fatty acid is greater in size making it difficult for it to interact with water quickly. Saturation and unsaturation plays an important role in determining melting point however. Fatty acids without double bonds have a higher melting point when compared to unsaturated compounds. This is because the double bond is very weak due to the kinks created by the double bond that causes the fatty acid to be less tightly packed thus the bonds in the fatty acid is easier to break.
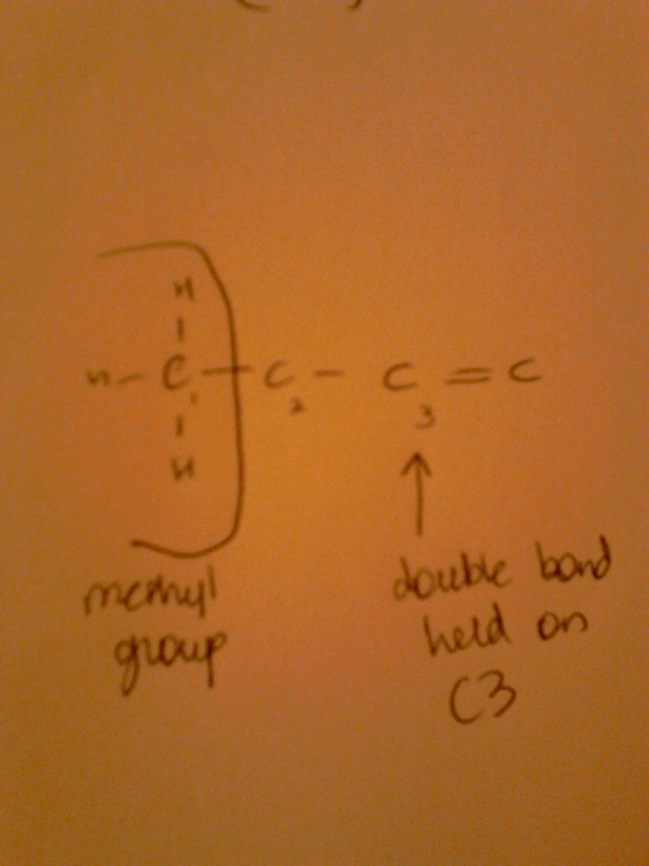
Essential fatty acids include omegas, found in for example, salmon and tuna. These are important for health issues and nutrition. Omega indicates the position of the first double bond from the methyl end of the fatty acid as seen below.
Unsaturated fatty acids undergo hydrogenation whereby the double bond is broken and 2 hydrogen atoms are added. This process is used in the production on butter for example and it increases shelf life.
To find the amount of unsaturation in a fatty acid is done by reacting it with iodine (iodine index) to determine the number of moles of carbon-carbon double bonds that react with iodine (1:1 ratio)
Trans fatty acids are deemed as the ‘bad’ lipids as they are formed when fatty acids are synthetically made by partially hydrogenating other polyunsaturated fatty acids. Some trans isomers may be formed. Trans fatty acids are found in some foods. Trans fats are considered undesirable as they have no kinks and are tightly packed. Due to this, trans fats cause a build up of plaque on the walls of arteries which can cause heart disease.
Fats require a greater degree of oxidation to become C0^2 and H20 than carbohydrates as carbohydrates already have 1 O for every C atom. Thus, more energy is provided as opposed to carbohydrates.
For TAG formation, 3 fatty acids bond to glycerol as glycerol has 3 OH groups in its molecule. 3 fatty acids bind to the glycerol molecule removing 3 moles of H2O. This forms an ester bond as seen in the image below. Hydrolysis of these bonds can lead to the formation of the 3 fatty acids and glycerol. These triglycerides can be broken down by a strong base for example NaOH to form a sodium salt and glycerol.
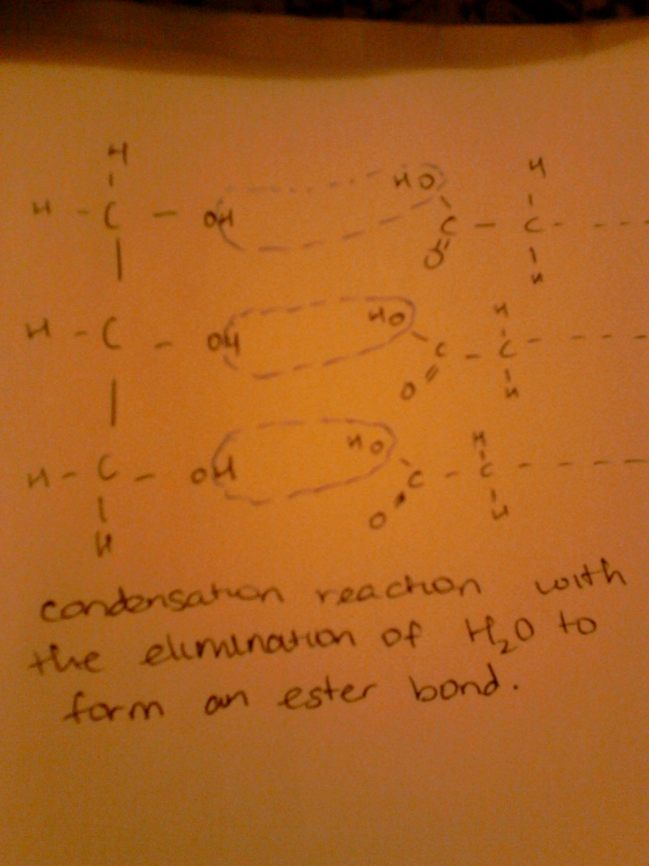
This concludes the reflection for lipids part 1, I hope that it was useful and will be an asset to you as you go on to lipids part 2 next week. 🙂
Posted in Uncategorized
Glycolysis Part 2
Gluconeogenesis
Gluconeogenesis is a metabolic pathway that takes place in the liver and to a smaller extent in the kidney and allows for the reformation of glucose from a substrate such as pyruvate. This process helps to maintain blood glucose levels and prevent them from falling too low and also the degradation of glycogen assists in sustaining blood glucose levels. This process takes place in many living organisms. Some important inputs in gluconeogenesis include glycerol and amino acids which are then catabolized to pyruvate, oxaloacetate or precursors of these. Preoteins present in muscle can also break down to provide amino acids which are then deaminated in the liver and changed to gluconeogenesis inputs. In this process, fructose-1,6-biphosphatase is inhibited by AMP.
The equation that summarizes gluconeogenesis is:-
2 pyruvate + 2 NADH + 4 ATP + 2 GTP ? glucose + 2 NAD+ + 4 ADP + 2 GDP + 6Pi
Feeder pathways for glycolysis
A feeder pathway for glycolysis is one that provides glucose or another starting substance into the pathway and this may include polysaccharides (glycogen and starch), disaccharides (maltose, lactose, trehalose and sucrose) or monosaccharides (fructose, mannose and galactose).
If a polysaccharide such as glycogen or starch are introduced into the pathway, three enzymes are needed which include glycogen phosphorylase, glucotransferase and phosphoglucomutase. Glycogen and starch are degraded by phosphorolysis.
If a disaccharide is introduced into the pathway, it cannot enter it as it is so therefore it must first be broken down to produce their monosaccharide units by their respective enzymes. (Lactose is a disaccharide and lactose intolerance is a condition that is caused by the loss of most or all lactase activity of the intestinal cells therefore making lit difficult to absorb or digest lactose. Some symptoms of lactose intolerance include abdominal cramps and diarrhea.)
Below shows the disaccharides that are broken down into monosaccharides and the respective enzymes involved:-
Maltose + H2O—————- 2 D-glucose
Maltase
Lactose + H2O—————- D-galactose + D-glucose
Lactase
Sucrose + H2O—————- D-fructose + D-glucose
Sucrase
Trehalose + H2O————- 2 D-glucose
Trehalase
Finally, hexoses can undergo glycolysis after being changed to a phosphorylated derivative and if a monosaccharide is introduced into the glycolytic pathway, the enzymes hexokinase, fructokinase, galactokinase and phosphomannose isomerase are needed to act on the sugars. Hexokinase acts on a different number of hexoses. Fructokinase is a liver enzyme and it catalyzes the phosphorylation of fructose. Galactokinase acts on glyceraldehyde 3-phosphate and D-galactose and is phosphorylated at Carbon 1 at the expense of ATP.
Metabolism of fructose
Fructose is a monosaccharide present in plants and can be metabolized by 2 ways.
- Fructokinase phosphorylates fructose in the liver and the fructose 1-phosphate pathway metabolizes it. Insulin does not regulate the uptake of fructose by the liver.
- Hexokinase phosphorylates fructose to fructose 6-phosphate in adipose tissue (muscle and kidney).
Metabolism of galactose
Galactose is a monosaccharide that is not as sweet as glucose and is an epimer of it. It goes into glycolysis via the galactose glucose intervention pathway. This pathway comprises of 4 steps and the 2nd enzyme, galactose 2-phosphate uridylyl transferase, is absent in the pathway and therefore toxic waste builds up and causes the disease, galactosemia.
Posted in Uncategorized














Recent Comments